Newly diagnosed ALK+ mNSCLC
ALECENSA was evaluated against crizotinib in a large study of newly diagnosed people with ALK+ mNSCLC
In this clinical study, ALECENSA was compared with crizotinib, which was the most commonly prescribed treatment for newly diagnosed people (people who hadn't been previously treated with an ALK inhibitor) with ALK+ mNSCLC. The study included people with and without ALK+ mNSCLC tumors that had spread to the brain at the start of study.
The effectiveness of ALECENSA was measured by 2 different groups of doctors. These groups of doctors are often used in clinical studies to make sure that there is a balanced review of results.
- One group are the Investigators, or INV. These are the doctors who treat and evaluate people in a study
- Another group is an Independent Review Committee, or IRC. These doctors do not treat the people in a study but evaluate and verify the results objectively
The people in the study receiving ALECENSA and crizotinib were evaluated at 2 different time points.
- The first assessment was performed as soon as results were available. These were the main results of the study and were used to support the approval of ALECENSA
- Like in many other studies, people continued to be monitored for a follow-up assessment
Learn more about the possible side effects of ALECENSA.
ALECENSA extended the median length of time people lived without ALK+ mNSCLC growing or spreading
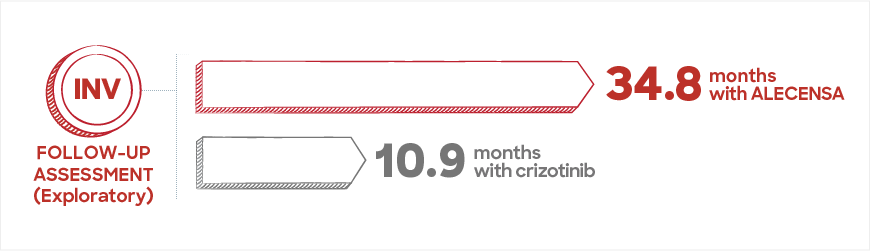
At the first assessment, the IRC found that, on average, people on ALECENSA lived more than twice as long without their ALK+ mNSCLC growing or spreading compared to people taking crizotinib. At this time, the Investigators were still collecting data.

The Investigators performed a follow-up assessment 10 months later once more data were available. The results from this assessment are from an exploratory analysis. This means that it was not specifically designed to find differences between ALECENSA and crizotinib. The Investigators found that the median time people taking ALECENSA lived without their disease spreading or growing was 34.8 months.
Learn more about the possible side effects of ALECENSA.
ALECENSA was able to shrink the size of tumors in nearly 80% of people with ALK+ mNSCLC
At the first assessment, the IRC evaluated the ability of ALECENSA to shrink the size of tumors in people with ALK+ mNSCLC.
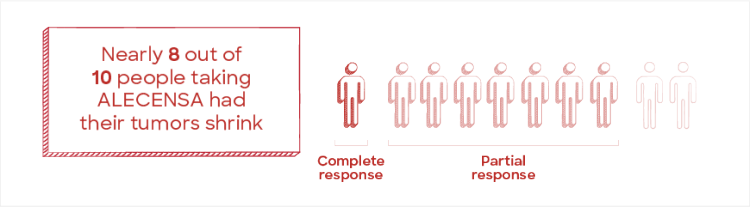
It is important to know that a complete response does not mean the cancer has been cured.
The results seen with crizotinib were similar to those seen with ALECENSA.
Of the people with ALK+ mNSCLC who had a reduction in tumor size, a response to treatment for 6 months or longer was seen in:
- 82% of people taking ALECENSA
- 57% of people taking crizotinib
Learn more about the possible side effects of ALECENSA.
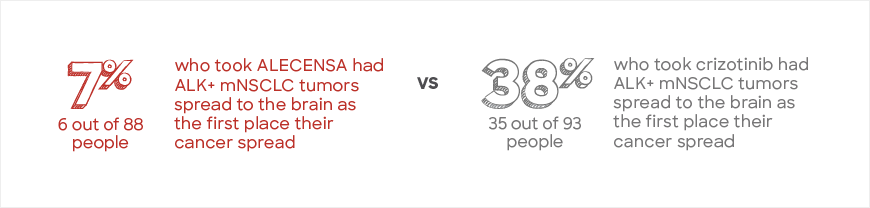
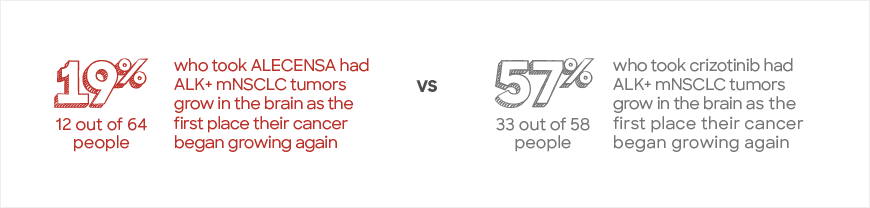
Fewer people who took ALECENSA experienced their ALK+ mNSCLC spreading to or growing in the brain
In the first assessment, the IRC found that fewer people who took ALECENSA experienced their ALK+ mNSCLC growing in or spreading to the brain as the first place their cancer spread. This included 122 people with and 181 people without ALK+ mNSCLC tumors that had spread to the brain at the start of the study.

In this first assessment, the IRC also separated people into 2 smaller groups as part of an exploratory analysis
In people without ALK+ mNSCLC-related brain tumors at the start of the study
In people with ALK+ mNSCLC-related brain tumors that were measurable on a
brain scan at the start of the study
People in this study who did not have ALK+ mNSCLC grow or spread to the brain first may still have had their cancer spread to other parts of the body.
Learn more about the possible side effects of ALECENSA.
ALECENSA was able to shrink the size of ALK+ mNSCLC tumors that had spread to the brain
At the first assessment, the IRC studied reduction in tumor size in people with ALK+ mNSCLC-related brain tumors that were visible on a brain scan at the start of the study, which was part of an exploratory analysis.

- 38% had their tumors disappear completely, also called a complete response
- 59% had their response last for more than 1 year

- 5% had a complete response
- 36% had their response last for more than 1 year
It is important to know that a complete response does not mean the cancer has been cured.
Learn more about the possible side effects of ALECENSA.
Overall survival data
Overall survival is the length of time from the start of treatment for a disease that people are still alive.

First assessment (main results to support the approval of ALECENSA):
Median overall survival was not reached for ALECENSA (meaning over half the patients were still alive at the time of the assessment).
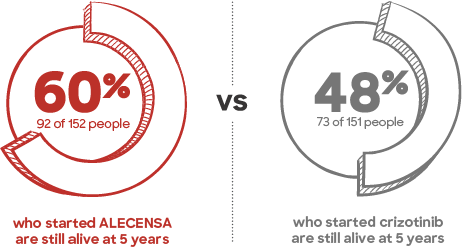
Follow-up assessment (exploratory analysis conducted 5 years after the last patient started treatment):
Median overall survival was still not reached because 60% of patients are still alive.
The results from this follow-up assessment were not used to support ALECENSA’s approval and not specifically designed to find differences between ALECENSA and crizotinib.

First assessment (main results to support the approval of ALECENSA):
Median overall survival was not reached for ALECENSA (meaning over half the patients were still alive at the time of the assessment).

Follow-up assessment (exploratory analysis conducted 5 years after the last patient started treatment):
Median overall survival was still not reached because 60% of patients are still alive.
The results from this follow-up assessment were not used to support ALECENSA’s approval and not specifically designed to find differences between ALECENSA and crizotinib.
Many patients taking ALECENSA reached a 5-year milestone.